Explain the Difference Between Ionic Compounds and Covalently Bonded Compounds
The ions stay together due to their. Covalent and ionic compounds can be differentiated easily because of their different physical properties based on the nature of their bonding.
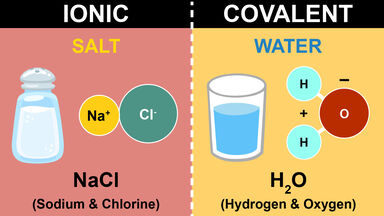
Difference Between Covalent And Ionic Bonds
Another clue is that only covalently bonded compounds use prefixes in the names.
. Covalent - A shared pair of electrons resulting in both atoms having full outer shells. A metal and a non-metal atoms in which one atom gives up an electron to another. All of the following pertain to nucleic acids.
Ionic compounds are formed when one or more electrons are transferred from one atom to another resulting in positive and negative ions. An ionic bond essentially donates an electron to the other atom participating in the bond while electrons in a covalent bond are shared equally between the atoms. Explain the differences between ionic and covalent bonds by answering the following questions.
Ionic bond also known as electrovalent bond is a type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Covalent bonding can only form between non-metallic elements. Ionic bond occurs due to the permanent transfer of one or more electrons from one atom to another.
Therefore their bonding pattern can be deemed as the key difference between ionic and. Ionic and covalent bonds are the major two types of chemical bonds that exist in compounds. The main reason for these differences is the difference in their bonding pattern.
A covalent bond occurs due to sharing of electrons between two atoms. Ionic is losing and gaining electrons Covalent is sharing the same electrons Ionic - One atom loses and electron the other gains one and two oppositely charged ions are produced which are attracted to each other. Here are some differences.
An ionic bond is a chemical bond between two dissimilar ie. Youll also see these as a gas or liquid rather than a solid like ionic bonds. In ionic bonding atoms transfer electrons to each other.
For each of the following fill in the blank with the correct responses. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. Ionic bonds require at least one electron donor and one electron acceptor.
Ionic bonds join metals to non-metals. This is not always the case however. Many differences can be noted between ionic and covalent compounds based on their macroscopic properties such as solubility in water electrical conductivity melting points and boiling points.
A second general feature of bonding also became apparent in the early days of chemistry. The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. A metal and a non-metal atoms in which one atom gives up an electron to another.
A given segment of the DNA molecule which contains the molecular coding for a specific protein to be synthesized is referred to as a _____. Ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent atoms bond covalently through the sharing of electrons between their outer shells. Learn vocabulary terms and more with flashcards games and other study tools.
Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. Bonding Differences Between Ionic and Covalent Molecular Compounds. An ionic bond is a chemical bond between two dissimilar ie.
At room temperature and normal atmospheric pressure covalent compounds may exist as a solid a liquid or a gas whereas ionic compounds exist only as solids. The strength of these chemical bonds can be loosely classified as strong bonds or weak bonds. It takes place been similar atoms ie.
This is the main difference between Ionic. But while covalent bonds might be a little weaker than ionic bonds they can form between the same elements. Ionic bonds occur between a metal and a non metal.
A covalent bond is another strong chemical bond. Start studying Chem unit 11. It was found that there are two large classes of compound that can be distinguished by their behaviour when dissolved in water.
How do ionic bonds differ from covalent bonds when it comes to what happens to the electrons of the atoms involved in each type of bond. As their names suggest ionic compounds are made of ionic bonds and covalent compounds are made of covalent bonds. In a covalent bond the two atoms come together to share the electron instead of an atom taking an electron from another.
Which bond ionic or covalent involves cations and anions. The main difference between the Ionic and Covalent Compounds is the methodology of formation. The only pure covalent bonds occur between identical atoms.
Ionic and covalent compounds. The two common examples of strong bonds are the ionic bonds and covalent bonds. One of the atoms in the bond shall lose an electron to initiate the bond to form an ionic compound while the Covalent compound is formed by sharing the electrons among the atoms.
How does the formation of cationanions in the bond. These compounds are so called because they dissolve to give. This kind of bonds occurs mainly.
Examples of ionic bonds are sodium chloride magnesium chloride magnesium oxide etc. Ionic bonding can only form between metals and non-metals. One class consists of electrolytes.
The ionic bond is the electrostatic force of attraction between two oppositely charged ions. The difference between ionic and covalent bond is that ionic bonds occur between atoms having very different electronegativities whereas covalent bonds occur between atoms with similar or very low electronegativity differences. A covalent bond is formed from the mutual sharing of one or more pairs of electrons between two atoms non-metals.
In general metallic elements tend to form ionic bonds and non-metallic elements tend to form covalent. In contrast atoms with the same electronegativity share electrons in covalent bonds because neither atom preferentially attracts or repels the shared electrons. Since covalent bonds are just sharing their stuff they arent as solid of a bond as ionic bonds.
This works best when the atoms in question have similar electronegativity values which is to say the strength with which they each attract other atoms and hold shared electrons is pretty equal.

Solved 5 Differences Between Ionic Compound And Covalent Compound Brainly In

Difference Between Ionic And Covalent Bonds Compare The Difference Between Similar Terms
What S The Difference Between An Ionic Bond And A Covalent Bond Quora
Comments
Post a Comment